This is the notes for Alkyne which is a important portion of Organic chemistry.
Alkynes
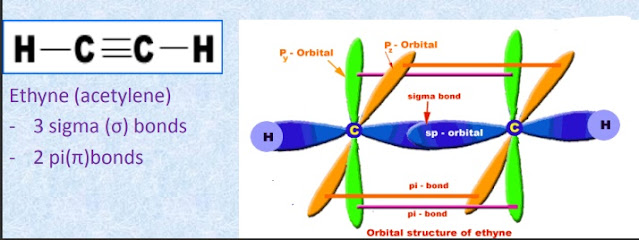
- Hydrocarbons containing carbon-carbon triple bonds are called alkynes.
- They have general formula CnH2n-2
- They are also called acetylenes and the first member is ethyne (acetylene)
General methods of preparation of Alkynes (ethyne)
1. By dehydrohalogenation of vicinal dihalides: Vicinal dihalides when heated with alc. potash give alkynes by dehydrohalogenation.
2. From calcium carbide:
CaC2(Calcium carbide) + 2H2O → Ca(OH)2 + C2H2(ethyne)
3. By direct combination of carbon and hydrogen(Berthelot’s synthesis)
4. Reaction of trihalogen derivative (iodoform or chloroform) with silver powder:
When trihaloalkane containing three halogen atoms on same carbon is heated with silver powder, alkyne is formed. Here dehalogenation takes place.
2CHI3(Iodoform) + 6Ag---Δ---≻ H-C≡C-H (Acetylene)+ 6AgI
2CHCl3(Chloroform) + 6Ag---Δ---≻H-C≡C-H(Acetylene) + 6AgCl
Chemical properties:
1. Addition of haloacids (HI,HBr,HCl):
H-C≡C-H(Ethyne) + HBr→CH2 =CHBr--- HBr→ CH3 -CHBr2(1,1-dibromoethane)
Alkynes react with two molecules of halogen acids in the presence of sunlight to give dihaloalkane. The addition takes place acc. to Markovnikov’s rule.
CH3 -C≡C-H(Propyne) + HBr→CH3 -CBr=CH2---HBr→CH3 -CBr2 -CH3(2,2-dibromopropane)
2. Addition of Ozone(ozonolysis): Ozone adds with acetylene across the triple bond to form an ozonide which on boiling with Zn dust and water gives glyoxal (ethane-1,2-dial).
3. Addition of water (catalytic hydration):
When ethyne is reacted with water in the presence of 1% HgSO4 and dil.H2 SO4 (10%) as catalyst at about 600C, vinyl alcohol is formed which undergoes rearrangement (tautomerisation) to give acetaldehyde. Addition follows Markovnikov’s rule.
4. Polymerization reaction:
Acetylene when passed through red hot Fe or Cu tube 3 molecules of acetylene undergoes polymerization to give benzene.
5. Acidic nature of ethyne: formation of acetylides
Ethyne is acidic in nature because the hydrogen atoms of ethyne can be replaced by metal like Na, Li, Cu, Ag, etc. forming metallic salts known as acetylides.
For eg:
I. Action with sodium metal
2H-C≡C-H(acetylene) + 2Na----Δ →2H-C≡CNa(monosodium acetylide) + H2
H-C≡CH(acetylene) + 2Na----Δ →NaC≡CNa(disodium acetylide) + H2
II. Action with ammoniacal silver nitrate solution (Tollen’s reagent):
Test of ethyne
Ammoniacal silver nitrate solution is called Tollen’s reagent. When ethyne is passed through Tollen’s reagent, a white ppt. of silver acetylide is formed. This is the test reaction of ethyne.
H-C≡C-H(Acetylene) + 2AgNO3 + 2NH4OH(Tollen’s reagent)---Δ →AgC≡CAg↓[Silver acetylide(white ppt.)]+ H2O + 2 NH4NO3
III. Action with ammoniacal cuprous chloride solution: Test of ethyne When ethyne is passed through ammoniacal cuprous chloride solution a red ppt. of copper acetylide is formed. This is the test reaction of ethyne.
H-C≡C-H(Acetylene) + Cu2Cl2 + NH4OH Δ CuC≡CCu↓[copper acetylide(red ppt.)]+ H2O + 2 NH4NO3
Uses of acetylene
- For producing oxy-acetylene flame for cutting and welding of metals
- For the artificial ripening of fruits and vegetables
- For the manufacture of organic compounds like acetaldehyde, ethyl alcohol, acetic acid, etc.
- As the starting material for the preparation of polymers such as polyethene, PVC, synthetic rubbers, etc.
- For making acetylene lamps for illumination.
1. Reaction with Br2/H2O or Br2/CCl4 :
a) In alkenes: Alkenes react with Br2 solution in H2O or CCl4 to give vicinal dihalides and discharge the reddish brown color of bromine.
b) In alkynes: Alkynes react with Br2 solution in H2O or CCl4 to give tetra-bromo alkanes and discharge the reddish brown color of bromine.
2. Oxidation by alkaline KMnO4 solution: Baeyer’s Test
The dil. alkaline solution of potassium permanganate is called Baeyer’s reagent.
a) In alkene: Alkene reacts with Baeyer’s reagent to give 1,2-diol and the pink color of the reagent is discharged. This reaction is called Baeyer’s test. This reaction is also called hydroxylation reaction.
b) In alkyne: Alkyne reacts with Baeyer’s reagent to give addition product and the pink color of the reagent is discharged. This reaction is called Baeyer’s test.
B] Kolbe electrolysis for the preparation of alkene
Electrolysis of concentrated aqueous solution of sodium or potassium salt of butanedioicacid (succinic acid) give Ethene (ethylene)at anode.
C] Kolbe electrolysis for the preparation of alkyne
Electrolysis of concentrated aqueous solution of sodium or potassium salt of fumaric acid or maleic acid give ethyne (acetylene) at anode.
Hope this will help you a lot.
For more, be in touch with eduguidenep.blogspot.com















Post a Comment